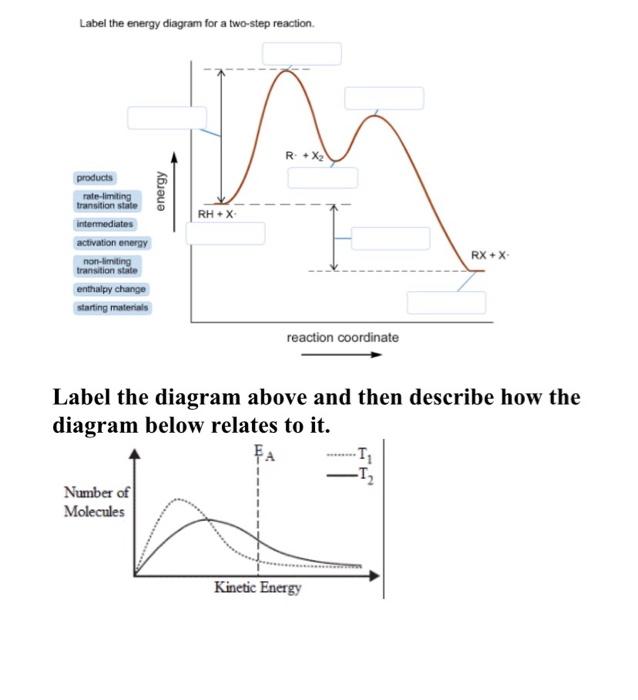
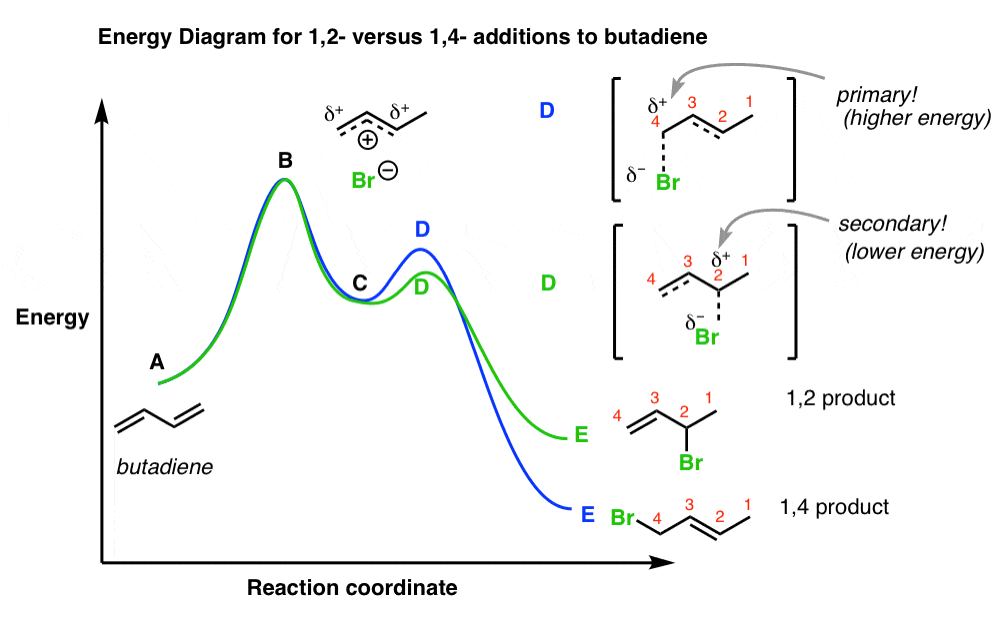
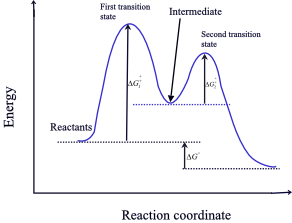
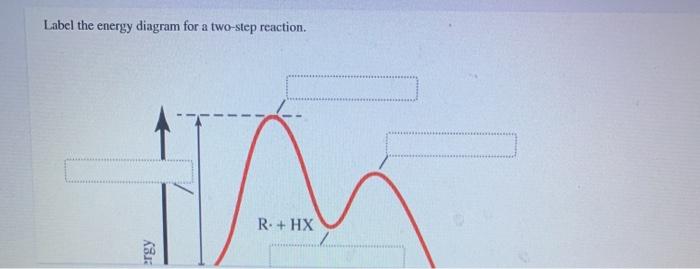
40 label the energy diagram for a two‑step reaction.
Graphene-Based Fiber Supercapacitors | Accounts of Materials … 01.08.2022 · ConspectusWearable electronics are smart devices that can be directly worn on the human body. Consumer-grade wearable devices (such as smart bracelets, watches, and glasses) are becoming increasingly popular. They provide continuous and reliable data analysis and guidance in our daily health monitoring and exercise activities. Meanwhile, professional … Solved Label the energy diagram for a two-step reaction. - Chegg Question: Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting transition state intermediates activation energy reaction coordinate This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (54 ratings)
Success Essays - Assisting students with assignments online Get 24⁄7 customer support help when you place a homework help service order with us. We will guide you on how to place your essay help, proofreading and editing your draft – fixing the grammar, spelling, or formatting of your paper easily and cheaply.
Label the energy diagram for a two‑step reaction.
Cold fusion - Wikipedia Cold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature.It would contrast starkly with the "hot" fusion that is known to take place naturally within stars and artificially in hydrogen bombs and prototype fusion reactors under immense pressure and at temperatures of millions of degrees, and be distinguished from muon … Labeling an Energy Diagram Diagram | Quizlet Labeling an Energy Diagram STUDY Learn Flashcards Write Spell Test PLAY Match Gravity Created by Corey_WilliamsonPLUS Terms in this set (9) Reactants Starting ingredients for Forward reaction Forward Activation Energy (Ea) Energy required to break the bonds between atoms for the FORWARD reaction Enthalpy (∆H) Answered: Kinetics: Draw a Potential energy… | bartleby Q: Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition… A: a. This is a one step reaction A gives B. This reaction is exothermic since B is lower than A.
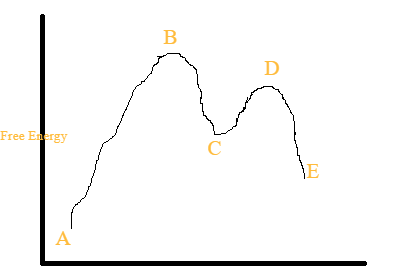
Label the energy diagram for a two‑step reaction.. Answered: Consider the following energy diagram… | bartleby How many steps are present in the reaction mechanism? d. Label each step of the mechanism as endothermic or exothermic. e. Label the overall reaction as endothermic or exothermic. B G Reaction coordinate Energy ... Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the ... 16.8: Electrochemical Corrosion - Chemistry LibreTexts 01.03.2022 · Corrosion can be defined as the deterioration of materials by chemical processes. Of these, the most important by far is electrochemical corrosion of metals, in which the oxidation process M → M + + e – is facilitated by the presence of a suitable electron acceptor, sometimes referred to in corrosion science as a depolarizer.. In a sense, corrosion can be viewed as the … Chapter 19, Problem 65P | bartleby Textbook solution for Physics for Scientists and Engineers with Modern Physics… 4th Edition Douglas C. Giancoli Chapter 19 Problem 65P. We have step-by-step solutions for your textbooks written by Bartleby experts! Upconverting nanoparticles - Wikipedia Physics. Photon upconversion belongs to a larger class of processes by which light incident on a material induces anti-Stokes emission. Multiple quanta of energy such as photons or phonons are absorbed, and a single photon with the summed energy is emitted. It is important to make the distinction between photon upconversion, where real metastable excited states allow for …
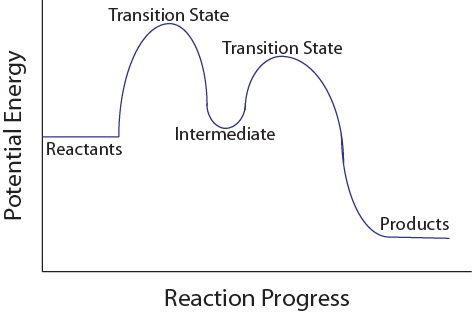
Label the energy diagram for a two-step reaction 19 Mar 2018 — An energy profile diagram is a theoretical representation that shows how the energy of the system changes during a chemical reaction. Some ... Answered: Draw a reaction energy diagram for a… | bartleby Label the intermediate, transition states, ΔH, and activation energies. Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate, transition states, ΔH, and activation energies. Question thumb_up 100% Programmable living assembly of materials by bacterial ... Dec 21, 2021 · After completing the degradation from PAR to PNP, the reaction of PNP to PAP was initiated by mixing 4.9 ml of reaction mixture in step 1 and 0.1 ml of 2 M NaBH 4. Supernatants were collected ... How to draw the potential energy diagram for this reaction? 1. Identify the general shape of the energy diagram. Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
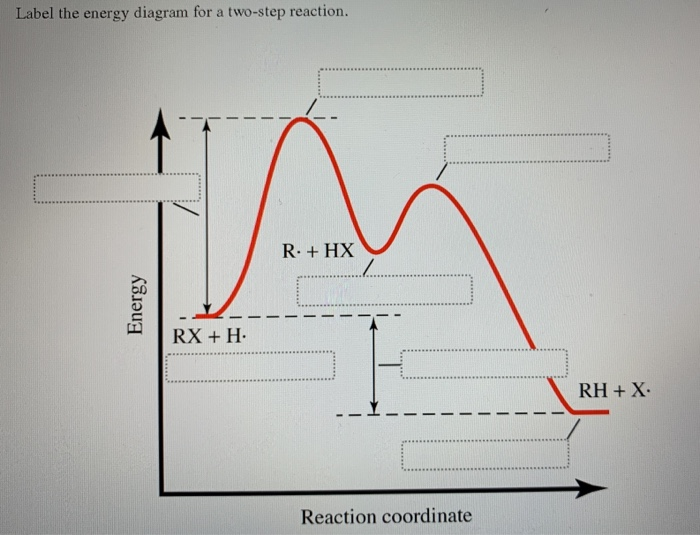
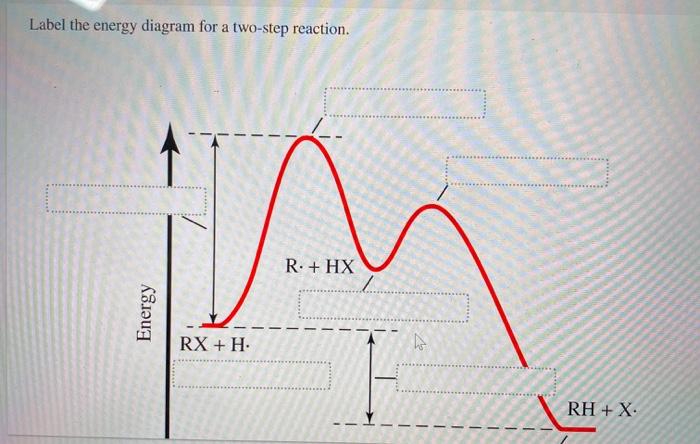
Label the energy diagram for a two-step reaction. - Chegg Answer to Solved Label the energy diagram for a two-step reaction. Inorganic Chemistry 4th edition, Catherine Housecroft A Pulse-Radiolysis and Theoretical Study of the Associated Reaction Mechanism. by Juan Costamagna and Guillermo Ferraudi. Download Free PDF Download PDF Download Free PDF View PDF. THE CHEMISTRY OF THE ACTINIDE AND TRANSACTINIDE ELEMENTS. by G N. Download Free PDF Download PDF Download Free PDF View PDF. Label the energy diagram for a two-step reaction. R - Chegg Question: Label the energy diagram for a two-step reaction. R: + HX ТЕУ Answer Bank rate-limiting transition state starting materials products non-limiting ... Engineering Chemistry by Jain & Jain - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
ACS Omega | Ahead of Print Articles ASAP (as soon as publishable) are posted online and available to view immediately after technical editing, formatting for publication, and author proofing.
Solved Label the energy diagram for a two-step reaction. R Question: Label the energy diagram for a two-step reaction. R: + HX Energy RX + H RH + X Reaction coordinate Answer Bank products enthalpy change activation ...
Krebs cycle / Citric acid cycle / TCA Cycle with steps and diagram 10.03.2022 · Image Source: Lehninger Principles of Biochemistry. The oxidative decarboxylation of pyruvate forms a link between glycolysis and the citric acid cycle. In this process, the pyruvate derived from glycolysis is oxidatively decarboxylated to acetyl CoA and CO 2 catalyzed by the pyruvate dehydrogenase complex in the mitochondrial matrix in eukaryotes and in the …
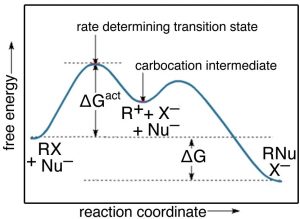
12.1: Introduction to Reactions of Metal Complexes The reaction coordinate is often drawn as the progress of a reaction from reactants (R) on the left to products (P) on the right.*. Examples of reaction coordinate diagrams for a one-step and a two-step reaction are shown in Figure 12.1. 1. Notice the difference in the reaction profile that depends on the number of reaction steps.
How to Draw & Label Enthalpy Diagrams - Study.com This enthalpy diagram has starting products, ending products, delta H, and activation energy labeled There are two different types of energy changes in reactions, endothermic and exothermic....
Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
Roller Coasters and Amusement Park Physics - Physics Classroom Determine the magnitude of any known forces and label on the free-body diagram. (For example, if the mass is given, then the F grav can be determined. And as another example, if there is no vertical acceleration, then it is known that the vertical forces or force components balance, allowing for the possible determination of one or more of the ...
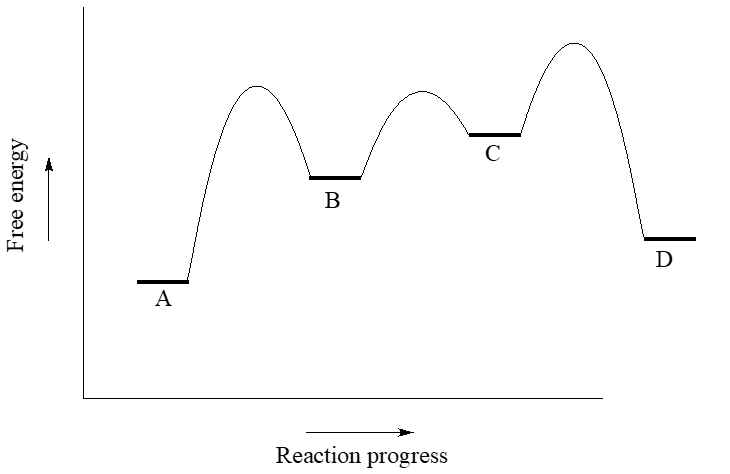
Answered: Draw a potential energy diagram given… | bartleby draw a potential energy diagram given the following information. - the reaction proceeds in three steps - the activation energy of step 2 is double that of step 1. - the activation energy of step 3 is double that of step 2 - the reaction is endothermic label all axes, the rate limiting step, the location of any intermediates and transition …
Answered: 1. In energy diagram 1, R represents… | bartleby A: We know that Energy profile diagram of two step reaction have to transition state and one… Q: Is CaCl2 (aq) + Na₂CO₃ (aq) → CaCO3 (s) + 2NaCl (aq) reversible or irreversible reaction A: This reaction is an irreversible reaction because in this reaction product Calcium carbonate (CaCO3…
Solved Label the energy diagram for a two-step reaction. R. Question: Label the energy diagram for a two-step reaction. R. + HX M Energy RX + H RH + X Answer Bank intermediates enthalpy change products starting materials ...

How to Draw Multi-Steps Energy Profile Diagrams: Reactant, Product, ∆H, Activation Energy, Slow Step
Solved Label the energy diagram for a two-step reaction. Question: Label the energy diagram for a two-step reaction. activation energy non-limiting transition state D products intermediates RX + H RH +X starting ...
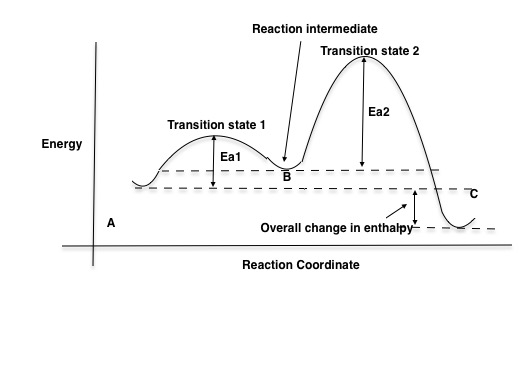
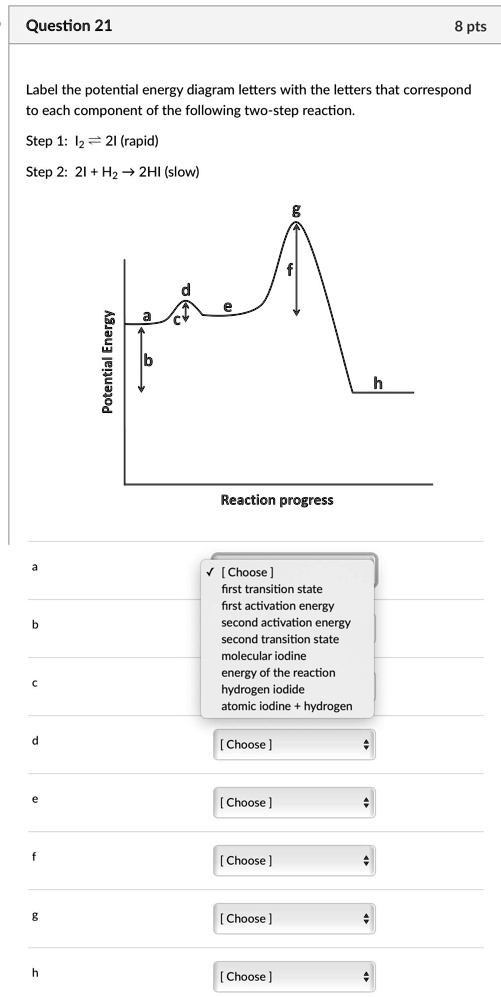
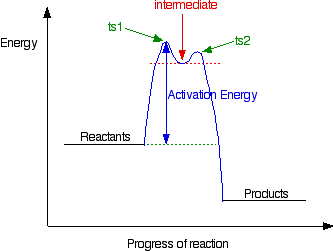
Multistep Reactions - Softschools.com The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
16.8: Electrochemical Corrosion - Chemistry LibreTexts Mar 01, 2022 · Figure \(\PageIndex{1}\): Corrosion is a two-step process. Figure \(\PageIndex{1}\): Electrochemical corrosion of iron. Corrosion often begins at a location (1) where the metal is under stress (at a bend or weld) or is isolated from the air (where two pieces of metal are joined or under a loosely-adhering paint film.)
How does the energy level diagram show this reaction is exothermic? Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
Solved Label the energy diagram for a two-step reaction. R. Question: Label the energy diagram for a two-step reaction. R. + HX Energy RX + H . RH + X Reaction coordinate Answer Bank enthalpy change intermediates ...
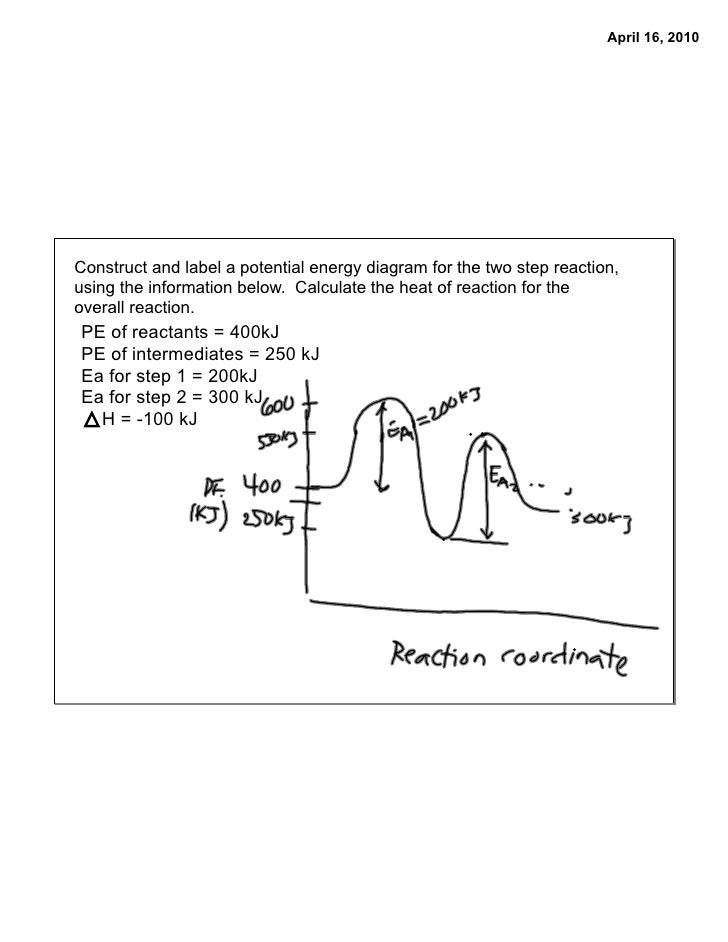
Energy Diagrams of Reactions | Fiveable To find the activation energy, you should be looking for two numbers: the potential energy of the reactants and the energy of the activated complex (the maximum point). (energy of activation complex) - (PEreactants) (100 kJ) - (40 kJ) = 60 kJ In other words, it takes 60 kJ of energy to complete the reaction.
Solved Label the energy diagram for a two-step reaction. R Question: Label the energy diagram for a two-step reaction. R: + HX Energy RX + H RH + X Reaction coordinate Reaction coordinate Answer Bank intermediates ...
Characterization techniques for nanoparticles: comparison and ... In this technique, a sample is exposed to low-energy gas ions, and the scattering and subsequent loss of energy of these ions can be related to the elemental composition of the outer layer surface. 147 High sensitivity LEIS (HS-LEIS) offers better sensitivity for the investigation of distinct atomic layers with an extensive reduction in surface ...
Mechanisms and Potential Energy Diagrams - Course Hero Summary A potential energy diagram for a two-step reaction is shown and labeled. Practice View the section on two-step reactions at the site below and then do the self-test (both buttons are at the top of the slide). Don't worry about - just consider it an indication of activation energy as is in the diagram above.
Energy Profiles (Energy Diagrams) Chemistry Tutorial - AUS-e-TUTE ⚛ An energy profile is a diagram representing the energy changes that take place during a chemical reaction. ⚛ Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. (2) On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products ...
Molecular convolutional neural networks with DNA regulatory 04.07.2022 · Fig. 5: The two-step classification approach based on a hierarchical network architecture for the recognition of 32 molecular patterns. a , Each input pattern is replaced by a pair of input ...
Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall.
SOLVED:Draw a reaction coordinate diagram for a two-step ... - Numerade here for the reaction. Coordinate diagram. Put two step verification in which the first step, Andrea Janek, the second step is extra, Janek. And the order reaction is and a sonic labels are re intense. Products, intermediates and chronic transition is start stairs.
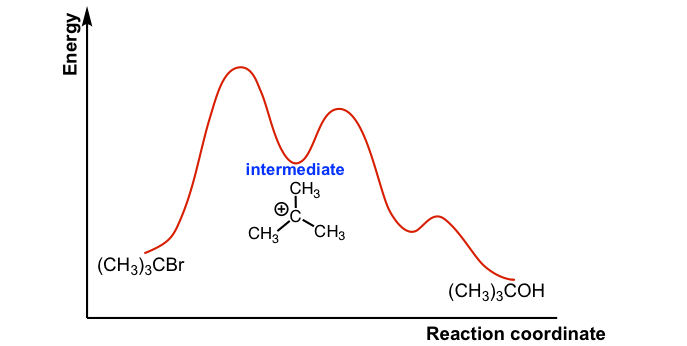
Draw an energy diagram for a two-step reaction, $$ A \righ | Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Draw an energy diagram for a two-step reaction, $$ A \rightarrow B \rightarrow C, $$ where the relative energy of these compounds is C < A < B, and the conversion of $$ B \rightarrow C $$ is rate-determining..
Reaction Mechanisms and Multistep Reactions - Chemistry LibreTexts Sketch out an activation energy diagram for a multistep mechanism involving a rate-determining step, and relate this to the activation energy of the overall reaction. Write the rate law expression for a two-step mechanism in which the rate constants have significantly different magnitudes.
Amusement Park Physics Determine the magnitude of any known forces and label on the free-body diagram. (For example, if the mass is given, then the F grav can be determined. And as another example, if there is no vertical acceleration, then it is known that the vertical forces or force components balance, allowing for the possible determination of one or more of the individual forces in the vertical …
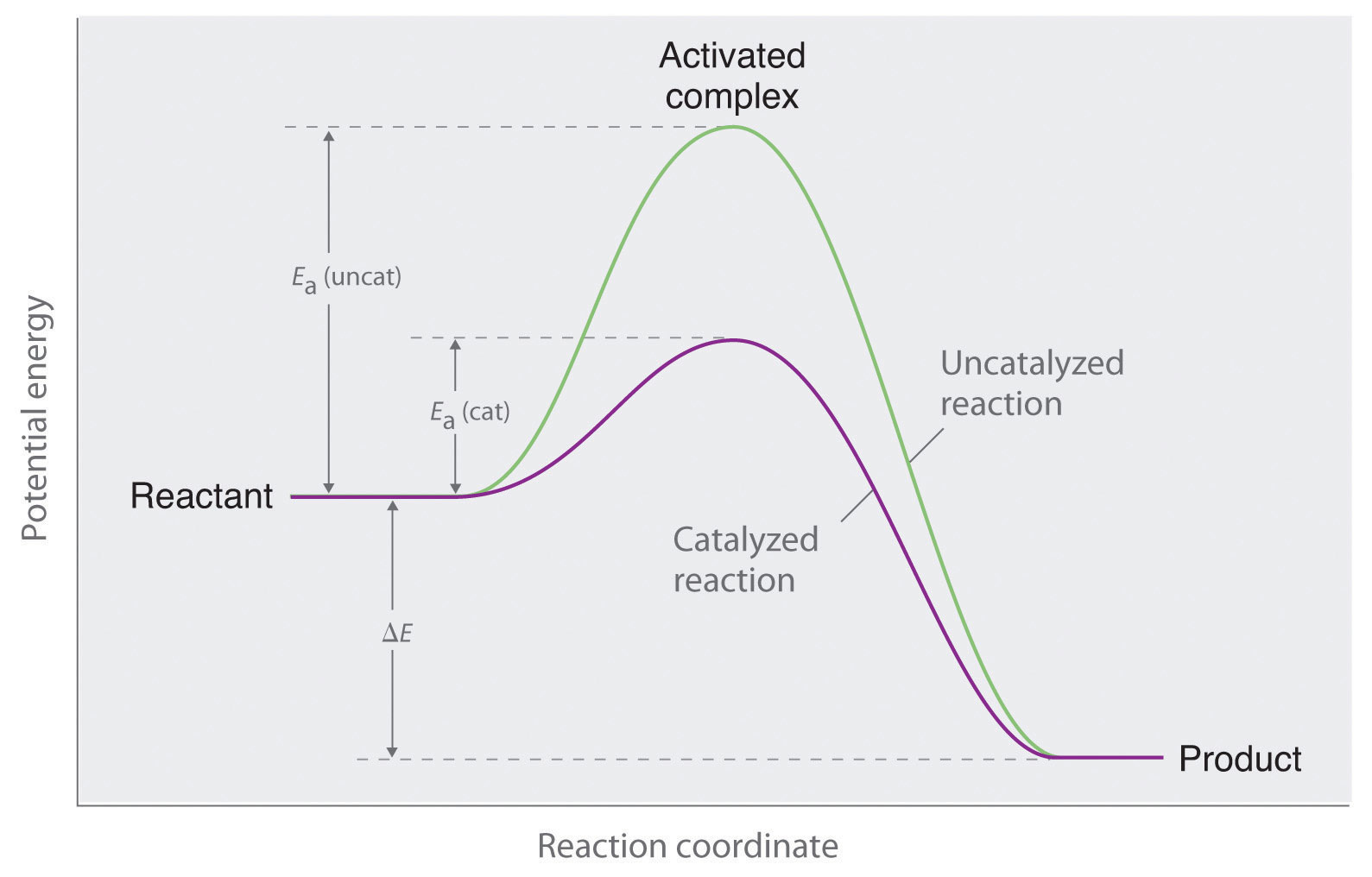
Potential Energy Diagrams Answer-->. KNO 3 NH 4 Cl NH 4 NO 3 NaCl. 6/04. 21 A catalyst increases the rate of a chemical reaction by. (1) lowering the activation energy of the reaction (2) lowering the potential energy of the products. (3) raising the temperature of the reactants (4) raising the concentration of the reactants. Answer-->.
(PDF) Engineering Chemistry by Jain & Jain - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
Answered: Kinetics: Draw a Potential energy… | bartleby Q: Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition… A: a. This is a one step reaction A gives B. This reaction is exothermic since B is lower than A.
Labeling an Energy Diagram Diagram | Quizlet Labeling an Energy Diagram STUDY Learn Flashcards Write Spell Test PLAY Match Gravity Created by Corey_WilliamsonPLUS Terms in this set (9) Reactants Starting ingredients for Forward reaction Forward Activation Energy (Ea) Energy required to break the bonds between atoms for the FORWARD reaction Enthalpy (∆H)
Cold fusion - Wikipedia Cold fusion is a hypothesized type of nuclear reaction that would occur at, or near, room temperature.It would contrast starkly with the "hot" fusion that is known to take place naturally within stars and artificially in hydrogen bombs and prototype fusion reactors under immense pressure and at temperatures of millions of degrees, and be distinguished from muon …













.docx_%255BCompatibility_Mode%255D_-_Word_(Product_Activation_Fai.png?revision=1&size=bestfit&width=641&height=416)





Post a Comment for "40 label the energy diagram for a two‑step reaction."