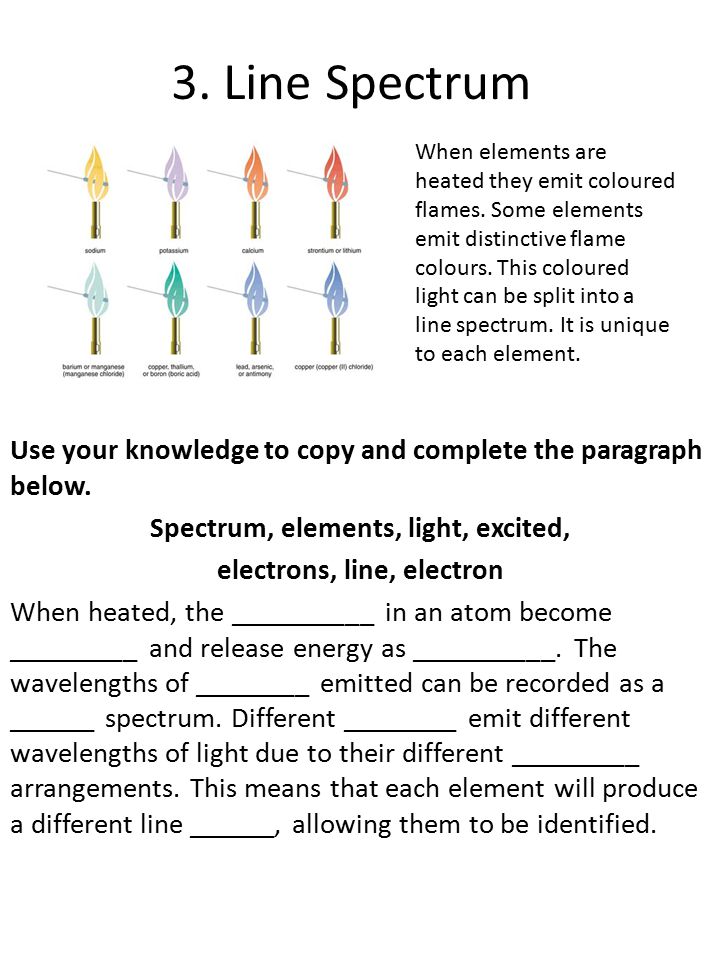
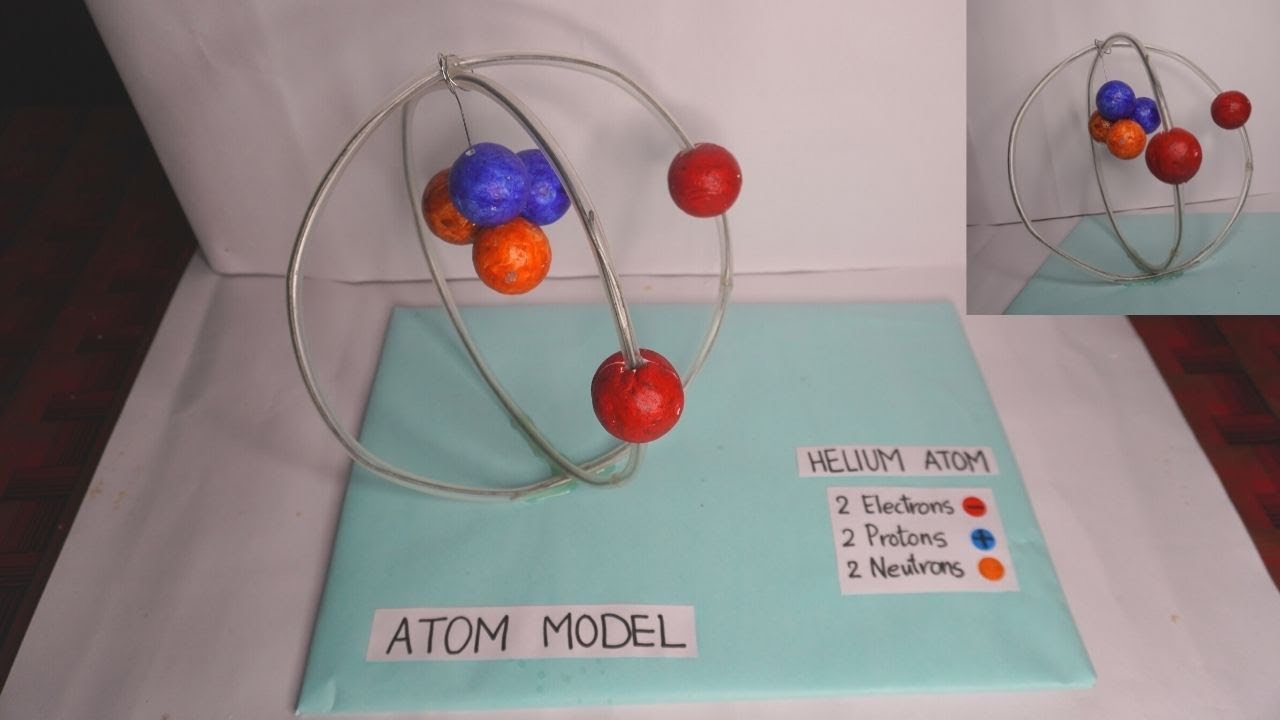
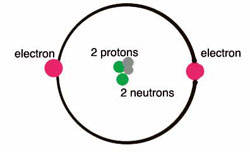
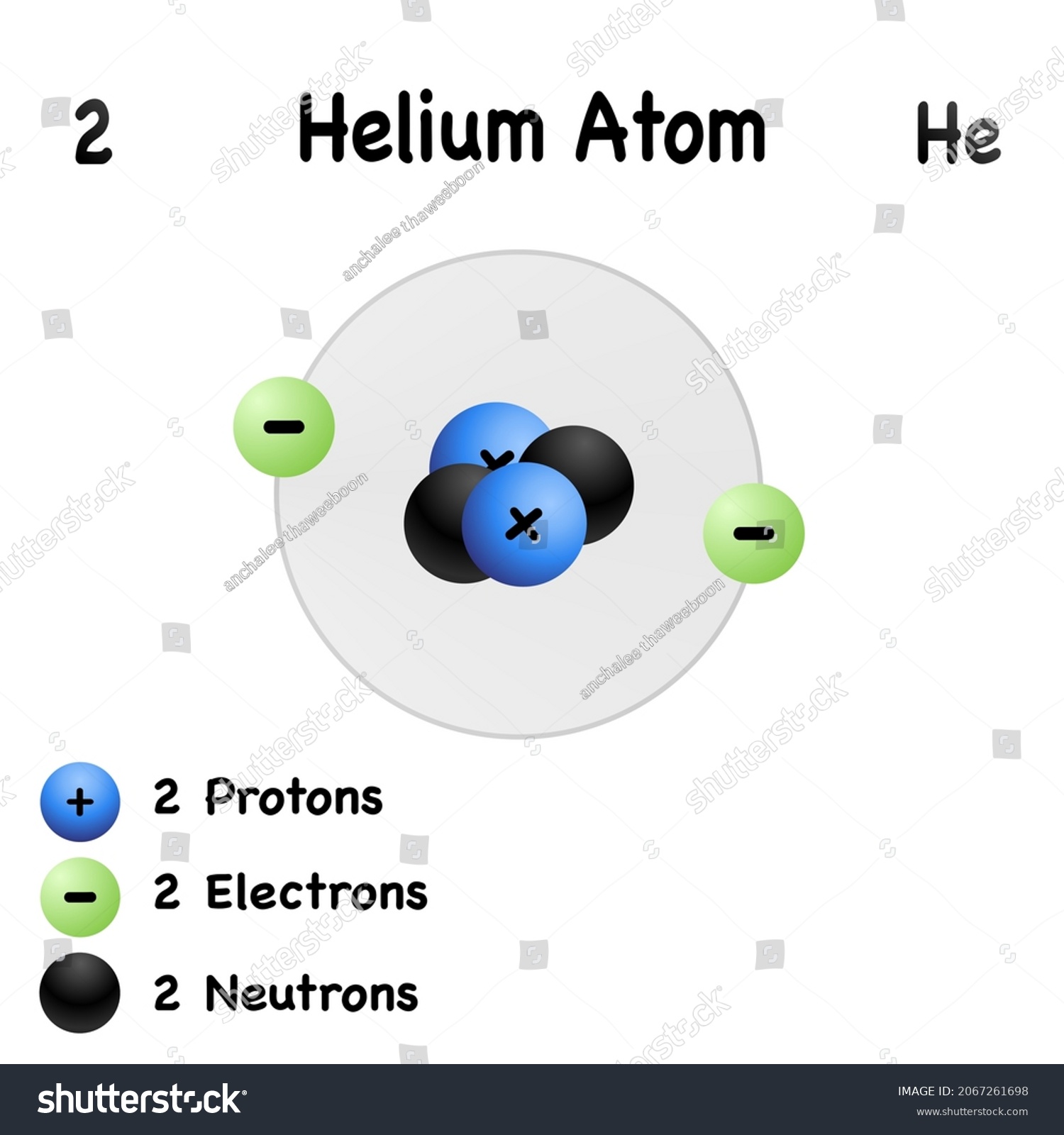
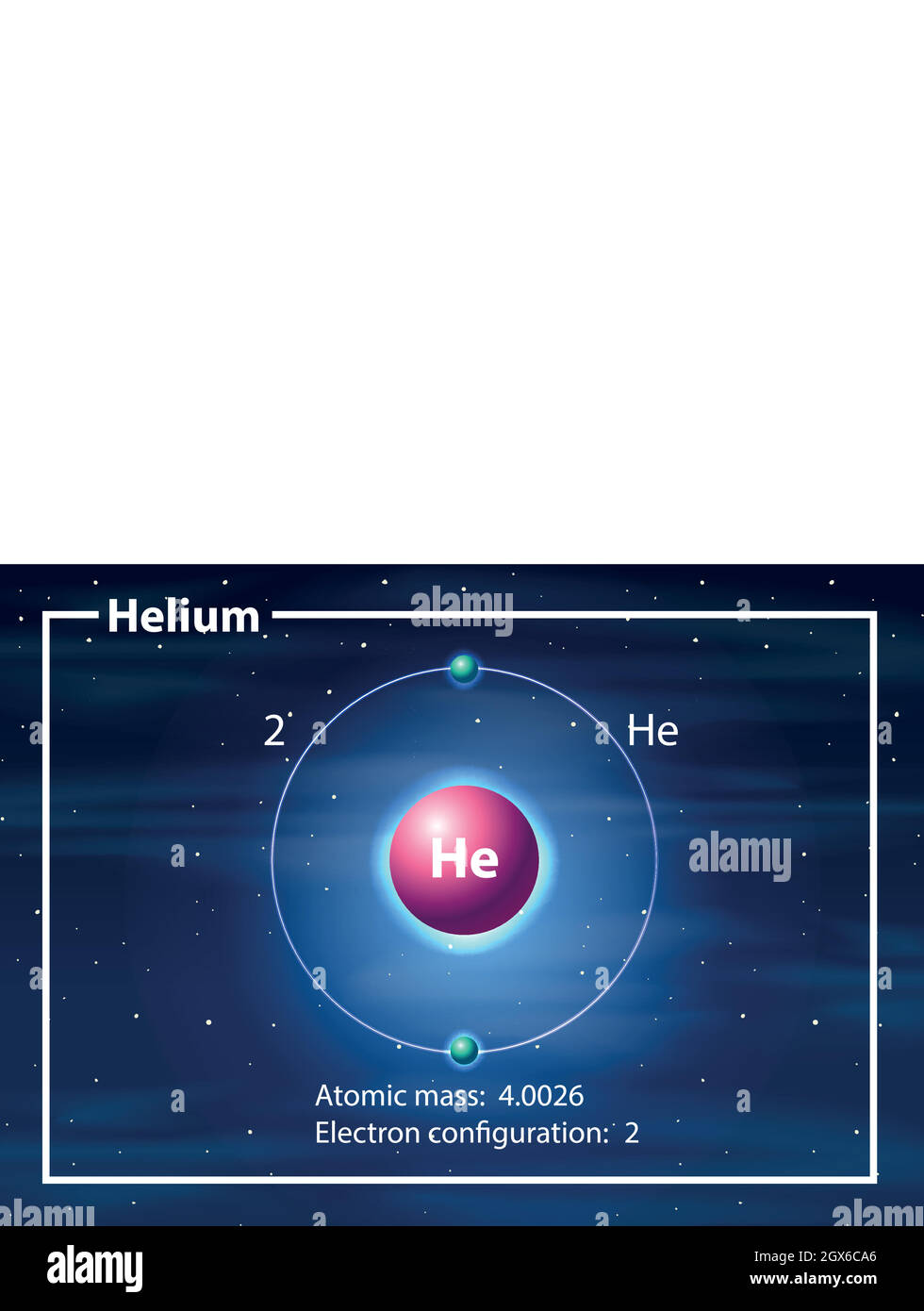
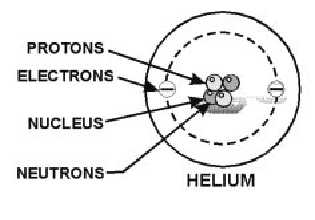
43 draw and label the parts of a helium atom
Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. Bohr developed this model of the atom which says the electrons revolve around the nucleus in a circular path called an orbit. The closest shell to the nucleus is called the "K shell" followed by the "L shell" then ... Drawing Atoms/Electronic Structure - Chemistrygcse.co.uk In the first shell the two electrons are placed at the top and bottom of the shell. In every other shell the first four electrons are placed at the four compass points (North, South, East, West) and then you repeat the process (i.e. the fifth, sixth, seventh and eighth electrons are paired).
Basic Chemistry Tutorial 2, Drawing Atoms - learn-biology Helium atoms have two protons, two neutrons, and two electrons. See if you can extend what we've learned so far to draw a diagram of helium. After you've drawn it, click "show the answer" to see if you drew it correctly. show the answer Make sure you've clicked "show the answer" and studied the diagram above before proceeding. 2. The Octet Rule

Draw and label the parts of a helium atom
Atomic Structure Quiz Flashcards | Quizlet Atomic Structure Quiz. Draw and Label the parts of a helium atom. Include the mass and charge of each subatomic particle. Should have two protons and two neutrons and 2 electrons and an electron cloud. protons: +, 1 amu neutrons: 0, 1 amu electrons: -1, 1/1840 amu. Helium - Periodic Table and Atomic Properties Helium is a chemical element with atomic number 2 which means there are 2 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. Structure of the atom - Atomic structure - AQA - BBC Bitesize Nucleus and shells An atom has a central nucleus. This is surrounded by electrons arranged in shells. The nucleus is tiny compared to the atom as a whole: the radius of an atom is about 0.1 nm (1 ×...
Draw and label the parts of a helium atom. Atoms and molecules - BBC Bitesize Water (H₂O) molecules contain one oxygen atom and two hydrogen atoms. Here, the oxygen atom is red and the hydrogen atoms are white. Ethanol molecules. Ethanol is a much larger molecule made of ... The periodic table, electron shells, and orbitals - Khan Academy Helium ( ), neon ( ), and argon ( ), as group 18 elements, have outer electron shells that are full or satisfy the octet rule. This makes them highly stable as single atoms. Because of their non-reactivity, they are called the inert gases or noble gases. Hydrogen ( ), lithium ( ), and sodium ( ), as group 1 elements, have just one electron in ... Lithium, atomic structure - Stock Image - Science Photo Library Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of lithium-7 (atomic number: 3), the most common isotope of the element lithium. The nucleus consists of 3 protons (red) and 4 neutrons (orange). Three electrons (white) include a relatively unstable electron in the outer shell (ring). The Structure of an Atom Explained With a Labeled Diagram According to his atom diagram, the atom has a small, positively charged nucleus in center. This nucleus carries the entire mass of the atom. Electrons revolve around the nucleus at a high-speed. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral.
PDF Modeling the Atom - VDOE helium has two protons in its nucleus. The number of protons in a particular atom determines the ... to draw their atom's "cross section." Ask them to label the parts of their drawing to show where we might expect to find the nucleus, a proton, a neutron, and an electron. 5. Have students separate the large candies into two color groups ... How to Draw a Helium Atom | Sciencing Most helium atoms contain two protons, two neutrons and two electrons. Draw a circle about 2 inches in diameter on a piece of paper. The circle represents the nucleus of a helium atom. Add two "+" symbols inside the circle to represent the two positively charged protons in a helium atom's nucleus. Rutherford model | Definition & Facts | Britannica A radioactive source emitting alpha particles (i.e., positively charged particles, identical to the helium atom nucleus and 7,000 times more massive than electrons) was enclosed within a protective lead shield. The radiation was focused into a narrow beam after passing through a slit in a lead screen. helium | Definition, Properties, Uses, & Facts | Britannica helium (He), chemical element, inert gas of Group 18 ( noble gases) of the periodic table. The second lightest element (only hydrogen is lighter), helium is a colourless, odourless, and tasteless gas that becomes liquid at −268.9 °C (−452 °F). The boiling and freezing points of helium are lower than those of any other known substance.
What Are The Parts Of An Atom? - Universe Today Our current model of the atom can be broken down into three constituents parts - protons, neutron, and electrons. Each of these parts has an associated charge, with protons carrying a positive... How to Draw an Atom! - YouTube If you want (or need) to draw a model of an atom, we'll show you how! Mass spectrometry - Wikipedia A mass spectrometer consists of three components: an ion source, a mass analyzer, and a detector. The ionizer converts a portion of the sample into ions. There is a wide variety of ionization techniques, depending on the phase (solid, liquid, gas) of the sample and the efficiency of various ionization mechanisms for the unknown species. Atom Diagrams: Electron Configurations of the Elements - ThoughtCo The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left. The upper right side shows the number of electrons in a neutral atom.
Energy Level Diagram For Hydrogen - Mini Physics The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.
Atomic Structure - Electrons, Protons, Neutrons and Atomic Models - BYJUS Atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. The negatively charged particles called electrons revolve around the centre of the nucleus.. The history of atomic structure and quantum mechanics dates back to the times of Democritus, the man who first proposed that matter is composed ...
ICAP2022 - The 27th International Conference on Atomic Physics In particular, the generation of photons and light-matter entangled states from the initial vacuum state can take place for a resonant time-modulation of the atomic transition frequency or the atom-field coupling strength, when the atom is directly coupled to the field via the dipole interaction (as described by the Quantum Rabi Model).
Helium-3 - Wikipedia Helium-3 (3 He see also helion) is a light, stable isotope of helium with two protons and one neutron (the most common isotope, helium-4, having two protons and two neutrons in contrast).Other than protium (ordinary hydrogen), helium-3 is the only stable isotope of any element with more protons than neutrons. Helium-3 was discovered in 1939. Helium-3 occurs as a primordial nuclide, escaping ...
Answered: among 1-18 which elements have the… | bartleby Sep 11, 2022 · Transcribed Image Text: Use the following equation to solve this problem: Zelt=Z-S where Zeff is the effective nuclear charge, Z is the actual nuclear charge, and S is the screening constant H Li Na Be Mg B Al C Si N P 0 S OF ва Ne D Ar 4
Build an Atom - Atoms | Atomic Structure | Isotope Symbols ... Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
How to Draw Lewis Structures - Albert Resources Examples for Drawing Lewis Dot Structure for Covalent Bonds . Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Reference the "How to Draw a Lewis Dot Structure" for a Step by Step guide. See the following Lewis dot structure diagrams for a few covalent compounds. Example 1.
Atom Diagram - Universe Today Basic chemistry explains the atom best. It states that the fundamental building block of matter is the atom. An atom consists of three main parts: protons, neutrons, and electrons. Protons have a ...
Engineering Chemistry by Jain & Jain - Academia.edu Enter the email address you signed up with and we'll email you a reset link.
Basic Model of the Atom - Atomic Theory - ThoughtCo The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged). Protons and neutrons form the atomic nucleus. Electrons are attracted to the protons in the nucleus, but are moving so quickly they fall toward it (orbit) rather than stick to protons.
Oxygen - Periodic Table and Atomic Properties Oxygen is a chemical element with atomic number 8 which means there are 8 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
PDF Label The Parts Of A Helium Atom - tbmc.edu.vn March 11th, 2018 - N ame Skills Worksheet Concept Review Section Atomic Structure Class Date 1 Draw and label the parts of a helium atom Include the mass and charge of Structure of the Atom grades 6 8 NYU April 25th, 2018 - Helium is an example of a noble inert gas It is not present in organisms because it is not chemically reactive
Chem Exam 1 Flashcards | Quizlet Draw a graph to show how the potential energy of the system changes with distance between the same two masses. ... (nucleus of the helium atom) passed straight through the gold foil. ... Therefore, only a small part of the volume of the atom contains the + charge. Draw a picture of Rutherford's model of the atom; what is wrong with it? A ...
How to draw Bohr diagram for Helium(He) atom - Topblogtenz Steps to draw the Bohr Model of Helium atom 1. Find the number of protons, electrons, and neutrons in the Helium atom Protons are the positively charged particles and neutrons are the uncharged particles, both these are constituents of the atom nuclei. Electrons are the negatively charged particles that orbit the nucleus of an atom
Structure of the atom - Atomic structure - AQA - BBC Bitesize Nucleus and shells An atom has a central nucleus. This is surrounded by electrons arranged in shells. The nucleus is tiny compared to the atom as a whole: the radius of an atom is about 0.1 nm (1 ×...
Helium - Periodic Table and Atomic Properties Helium is a chemical element with atomic number 2 which means there are 2 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.
Atomic Structure Quiz Flashcards | Quizlet Atomic Structure Quiz. Draw and Label the parts of a helium atom. Include the mass and charge of each subatomic particle. Should have two protons and two neutrons and 2 electrons and an electron cloud. protons: +, 1 amu neutrons: 0, 1 amu electrons: -1, 1/1840 amu.






























Post a Comment for "43 draw and label the parts of a helium atom"